Click arrows to read more.
Click arrows to read more.
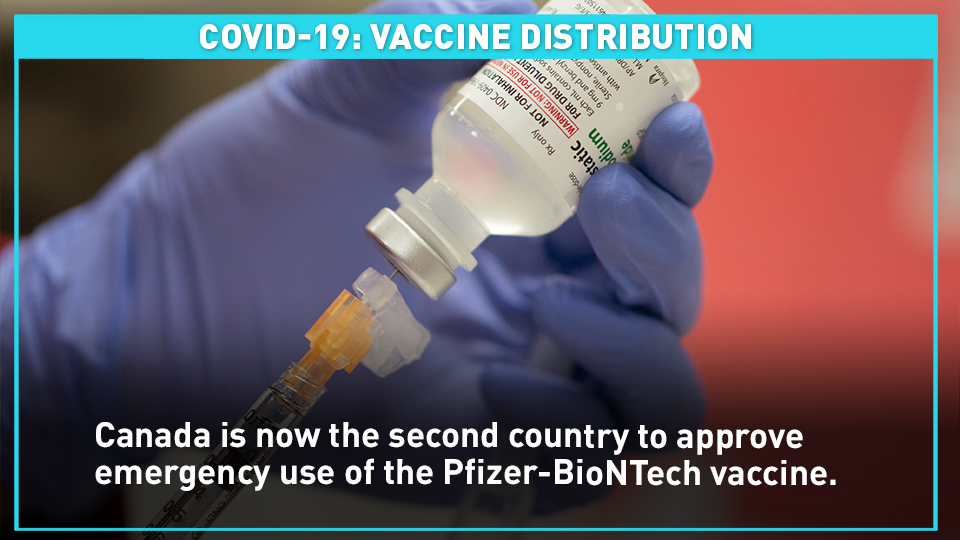
Canada is now the second country to approve emergency use of the Pfizer-BioNTech vaccine.
After a two-month review of Pfizer's clinical trial data, Health Canada announced the approval on Wednesday.
Canada's first doses are expected to arrive next week, according to Prime Minister Justin Trudeau.
A day after the first COVID-19 immunizations were given in the UK, health officials are warning people with severe allergic reactions to not take the vaccine.
Out of the thousands of vaccinations given on Tuesday, two healthcare workers experienced adverse reactions.
The workers have a history of allergic reactions and carry adrenaline auto injectors.
Before immunizations can begin in the U.S., the Pfizer vaccine is awaiting emergency use approval for high-risk groups.
The U.S. Food and Drug Administration (FDA) has declared Pfizer’s vaccine safe and effective, and indicated they could approve the vaccine when it meets on Thursday.
If emergency authorization is granted, vaccinations could begin next week, CNBC reports.
Because of limited supplies, a U.S. federal advisory panel has recommended giving the vaccine first to those at highest risk of infection: healthcare workers and the residents and staff in long-term care facilities.
But as richer countries prepare for vaccine arrival and distribution, some are warning, 90% of people in poor countries won’t get vaccinated next year.
Almost 70 countries fall into this category, according to the People's Vaccine Alliance, a group of organizations advocating for free and fair distribution of the vaccine worldwide.
The alliance is calling on companies working on a COVID-19 vaccine to share their information through the World Health Organization (WHO), so that more safe vaccines can be made and distributed.
Even though rich countries represent around 14% of the world's population, these countries already have bought 53% of the most promising vaccine candidates, according to Amnesty International.
Canada currently has bought the most vaccines. When ready, their supply would be able to vaccinate each Canadian five times.
The Pfizer-BioNTech vaccine needs to be transported and stored at -70 degrees Celsius (-94°F).
This requires dry ice, and several U.S. states are rushing to make sure they have enough.
Pfizer says, with dry ice, the vaccine can be stored for up to 30 days but must be re-iced every five days.
Check out The China Report, our new weekly newsletter. Subscribe here!

